Background:
The exhaustion and diminished persistence of CAR-T cells primarily affect clinical efficacy and long-term outcomes. Epigenetic is an innate mechanism in regulating the biological function CAR-T cells. We previously dissected comprehensive dynamic changes of chromatin accessibility and transcription factors during CAR-T cell exhaustion (Jiang P, et al. Leukemia. 2022 Nov;36(11):2656-2668). Therefore, combined application with epigenetic drugs have much therapeutic potential.
Methods:
We initially performed an extensive screening among 370 epigenetic drugs to select candidates that could resist exhaustion and maintain phenotype of memory subsets, and thoroughly verify their effects on CAR-T cells in vitro. Next, we conducted multi-omics analysis comprising RNA-seq, ATAC-seq, and CUT&Tag-seq to explore underlying regulative mechanisms of epigenetic manipulation by epi-drugs. In the end, we used preclinical B-ALL NSG mouse model to verify anti-tumor activities of drug-treated CAR-T cells in vivo.
Results:
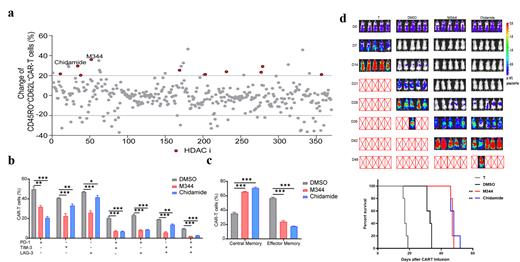
Among those screened epigenetic drugs, we identified two histone deacetylase inhibitors (HDACi), Chidamide and M344, could best promote maintenance of central memory subsets and resistance to exhaustion of CAR-T cells, with enhanced anti-leukemia activities in vitro (Figure 1a-c). Chidamide and M344 inhibited enzymatic activity and expression of HDAC1, which elevated the acetylation level of histone H3, particularly at H3K27 in CD19 CAR-T cells. Genetic knock-down of HDAC1 could partially reflect the phenotypic mimicry by Chidamide and M344 on CAR-T cells. By combining multi-omics data from bulk RNA-seq, ATAC-seq, and H3K27ac-targeted CUT&Tag-seq in Chidamide-or M344-treated CAR-T cells, we identified distinct patterns of epigenetic reprogramming, and enrichment of genes associated with exhaustion, as well as regulation of cellular differentiation and formation of memory subsets. In addition, analysis of motif and interaction network among transcription factors revealed altered regulation of gene transcription (data not shown). Finally, either Chidamide-pretreated CAR-T cells or in vivo administration of Chidamide after CAR-T cells infusion had exhibited enhanced and persistent tumor clearance in preclinical B-ALL NSG mouse model (Figure 1d).
Conclusion:
we conducted comprehensive screening of 370 epigenetic drugs and identified that administration of HDACi, particularly Chidamide and M344, could induce significantly enhanced anti-tumor function with maintenance of memory phenotype and reduced exhaustion in CAR-T cells through epigenetic reprogramming and altered regulation of gene transcription, which provided with basis and therapeutic targets for the rational combination of CAR-T cell therapy and epigenetic drugs in patients with hematological malignancies.
Figure legends:
Figure1. Screening of 370 epigenetic drugs identified M344 and Chidamide that enhanced maintenance of memory phenotype, resistance to exhaustion, and ability of tumor clearance in CAR-T cells. (a) The scatterplot of screening 370 compounds based on relative CD45RO +CD62L + proportion of central memory in treated CAR-T cell, showing of compared with DMSO control group by flow cytometry. Red indicates an increase in the proportion of memory CAR-T cells, while blue represents a decrease. (b) Frequency of co-expression of exhaustion-related inhibitory receptors (LAG-3, TIM-3, and PD-1) in DMSO or HDACi-treated groups. Data presented as mean ± SEM of independent donors (n=3 or more). (c) Flow cytometric analysis of central memory and effector memory subsets in CAR-T cells in each group. Data presented as mean ± SEM of independent donors (n=3 or more). (d) Bioluminescent imaging of tumor clearance in B-ALL NSG mouse. ‘X’ represents dead subject (end point). Survival of B-ALL NSG mice i.v. infused with T cells or treated CAR-T cells. Statistical analysis by Mantel-Cox test. Data presented as pooled from independent experiments (n=2).
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal